Cell therapies, Living medicines
Using antibodies to understand the re-writing of therapeutic paradigms
Cell therapies currently exist in an instant of deceptive potential— obscured by a short-term trough of disillusionment. While skeptics focus on manufacturing complexity, reimbursement challenges, and uncertain dosing regimens, these obstacles mirror the early hurdles faced by antibody therapies decades ago (discussed at length below). What some fail to recognize are the converging technological breakthroughs on the horizon: closed-loop manufacturing systems, microfluidic platforms, and in vivo transdifferentiation and cell expansion techniques that could move the needle on manufacturing efficiency and therapeutic efficacy.
At Compound, we believe these innovations will drive adoption and transform cell therapies from specialized treatments to mainstream therapeutic options. By reframing disease at the cellular level—the fundamental building block of life— the field is establishing an entirely new paradigm of medicine that will ultimately render today's skepticism as shortsighted as the initial doubts about antibody therapies in the 1980s.
A brief history of antibodies
Antibodies were used broadly in diagnostics (even for consumers - check out this ovulation prediction test from the ‘80’s) for years before antibodies were an established therapeutic modality. But the uncertainty around in-human safety and manufacturing lengthened the time from discovery to scaled therapeutic implementation.
The first monoclonal antibody approval was in 1987 - Orthoclone for kidney transplant rejection. Many patients were allergic to this drug due to the mouse origin of the antibody, stalling enthusiasm for investment and company building around expanded clinical development.
But scientists were emboldened and the early difficulty of Orthoclone spurred development of human-mouse antibody chimeras, working towards humanized antibodies which didn’t elicit negative immune response. Technology convergences made antibody engineering and targeting much more scaled and potent. The essential underlying technologies were recombinant DNA technology, phage display assays, and humanized mice and crucial basic science uncovering the importance of (now blockbuster) targets that drive large disease areas (TNFα, HER2, and CD20) . Through this technology readiness progression, Chinese hamster ovary (CHO) cell lines were selected as the preferred chassis for antibody production given their high expression of transgenes, fast growth in chemically-defined media, and similar post-translational modifications to humans.
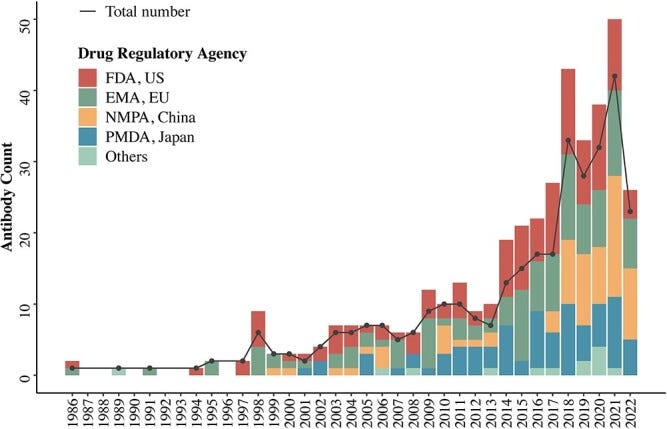
Though it took a decade of improvement from the first approved antibody therapy to many approvals today we see upwards of 40 approvals per year globally, with close to half of approvals being antibodies.
Back to cell therapies
The lesson from antibodies is it took about 10 years from first approval to scaled therapeutic utility, and 20 years from then to compete with small molecules in approval numbers. While we think this innovation window will shorten in the future, we’re thankfully 15 years post the first cell therapy approval. Provenge was the first cell therapy approval in 2010 which used autologous immune (dendritic) cells for the treatment of prostate cancer. In recent years there’s been a steady drip of approvals with thousands of novel cell therapies currently in clinical trials. This emergence of clinical trials in cell therapies tells us that 14 approvals are soon to be 10’s if not 100’s more in the upcoming years.
When looking at the cell therapy approvals, it’s incredible to see the diversity of cell types and use cases of the therapies in the market today. From the cases we all know such as using engineered T-cells to treat cancer (Kymriah, Yescarta, Aucatzyl, Carvykti, Tecartus, Breyanzi), autologous fibroblasts to decrease wrinkles (laViv), autologous dendritic cells for prostate cancer (Provenge), allogeneic pancreatic islets for Type I diabetes (Lantidra), allogeneic hematopoietic progenitor cell therapy (Omisirge), allogeneic bone marrow-derived mesenchymal stromal cell (MSC) therapy for graft versus host disease (Ryoncil), and engineered a melanoma-associated antigen A T-cell for sarcoma (Tecelra). The breadth and depth of types of use-cases is reminiscent of the exploding variants of antibody-based technologies (bispecifics, trispecifics, nanobodies, antibody drug conjugates, and many more.)
Fun fact: In 2011, laViv was approved to use autologous cell therapy (for connective tissues cells, fibroblasts) for the reduction in nasal-labial folds for beauty enhancement. Though these companies weren’t striking commercial successes (Dendreon now is primarily a contracting manufacturing organization (CMO) for cell therapies and Fibrocell was bought by a rare disease holding company Castle Rock in 2019) they broke the ice in the field.The need for therapies in some indications like ALS is so high that trials that don’t meet clinical endpoints can still progress in the clinic via Special Protocol Assessment (SPA). NurOwn, a mesenchymal stem cell therapy, from Brainstorm didn’t meet a primary endpoint but through an SPA was furthered to Phase IIIb trials, primarily due to a subset of early onset patients showing clinically significant results compared to placebo.
Fun fact: In high unmet need diseases SPA’s are ways for interest groups to push therapies through. For example, Tofersen, an ALS antisense oligonucleotide (ASO) from Biogen, did not meet primary endpoints but was still approved because a pathogenic biomarker of a neurofilament light chain was decreased in the treatment group. Tofersen is also covered by Medicare Advantage.Dark alchemy of engineering new therapeutic areas
As seen above in the cell therapy approvals, the main indication space to-date is in using cells for cancer therapy. While important and growing, approval of Lantrida for Type I diabetes opens up the frontier of indication space for cell therapies.
Early clinical studies show that edited islet cells could enable Type I diabetes treatment without immunosuppressive drugs - a massive breakthrough for Sana Biotechnologies. Aspect Bio’s platform using microfluidics and biomaterials to print islet cells and human hepatocytes has potential in Type I and II diabetes and liver failure. The liver failure indication challenges the paradigm of xenotransplantations for organ failure though data will show the feasibility of their approach.
Separately, the advancement of central nervous system (CNS) cell therapies is undeniable. Bluerock Therapeutics (Bayer) moved directly from Phase I to Phase III off the back of their patients’ Parkinson’s improvement via dopaminergic neuron implantation. Neurona Therapeutic’s cell therapy for epilepsy, showing their implanted inhibitory neurons decrease debilitating seizures by >90%. Reprogrammed stem cells to treat damaged corneas showed significant sight improvement in three patients, opening up future treatment potential in diseases such as limbal stem-cell deficiency. Preclinical work on spinal cord injury with cell replacements with deep cortical neurons and with hydrogels can be extrapolated to the potential of making paralyzed patients walk again.
There’s an eye-watering amount of scientific tangents academics are exploring related areas to cell replacement. Some of the more visionary tangents include transdifferentiation. The simple way to think about this is converting a cancer cell to an immune cell *inside a person*. Recent work demonstrates the transformation of converting cancer cells into tumor-reprogrammed antigen-presenting cells in murine leukemia models. There’s increased complexity of in vivo editing specifically around targeting and dosing which is a nontrivial problem which have been critiques of in vivo editing platforms for cancer cell therapy. Despite the difficulty in *getting* this new modality to work, it’s important to understand breakthroughs in this field. Other stunning transdifferentiation examples include:
Fun facts: The diversity and creativity of cell therapies and cells for *new* use-cases prove that life is stranger than fiction. In this study muscle cells patches differentiated from stem cells fortified a weak heart to give the patient time before a heart transplant. Another shows the importance of biobanking stem cells to save endangered animals. Yet another study banks stem cells from centigenarians to derive secrets to long-life.The upshot of cell therapies is eventual tissue replacement. This field has had more progress than people realize with de novo muscle formation in vitro, approval of ‘fake’ arteries with SYMVESS, and ISPC to 3D printing using bioreactor systems. Historically I’ve had some skepticism about bioprinting but this progress really changes my priors for the feasibility of eventual bio-printed organs. We’re emboldened by government interest in projects which are funding companies working on brain replacement tech. The leader of this project, Jean Herbert, wildly did in vivo transplantation platform that can support the development of functional neocortical tissue by allowing dissociated embryonic mouse brain cells to differentiate, form layered structures, establish vascular networks, and integrate functionally with the host brain when transplanted into lesioned adult mouse cortex.
The manufacturing elephant in the room
Just as technology convergence of humanized mice, recombinant DNA tech, and CHO lines, unlocked the mass production of antibodies, today's manufacturing innovations are changing the traditional barriers of cell therapy production. Companies like Cellares are redefining what's possible with their integrated development and manufacturing organization (IDMO) model, achieving tenfold productivity increases and 75% reduction in failure rates - numbers that would have seemed impossible in the early days of cell therapy, much like the initial scaling challenges faced by antibody manufacturers. The industry's trajectory is accelerating beyond traditional manufacturing constraints with a similar kind of technological convergence that enabled antibody engineering to become more scaled and potent. Not only is closed-loop manufacturing useful but strides in miniaturization of cell production with microfluidics and closed loop cell manipulation can be extrapolated to lower CapEx and COGs methods of cell therapy production. Molecular techniques such as cell differentiation gating with microRNA sensors have potential to decrease resultant heterogeneity. Another exciting trend is the emergence of in vivo manufacturing approaches; Novartis's T-Charge and Umoja's Vivovec are pioneering methods to expand engineered cells within patients' bodies. Cell therapy’s manufacturing evolution suggests we're accelerating the playbook with a substrate (cells) that are more central to health and disease.
Though cell therapies are currently ‘out’ of favor on the growth side, history tells us that troughs of disillusionment are temporary if the modality works (as was the case with antibodies). We couldn’t be more compelled by their potential for cell and eventual tissue therapies.
Please reach out if you’re working on the manufacturing or therapeutics area of this field!






I would welcome a discussion on off the shelf allo versus “shallow”-geneic, as in my Letter to Shareholders; https://investor.lineagecell.com/news-releases/news-release-details/lineage-cell-therapeutics-issues-letter-stockholders. Bculley@lineagecell.com
Very interesting, thanks for the write-up!