DNA synthesis - a technical primer
An engineering, not a biology problem
**Nima and Shelby contributed equally to this piece.
TL;DR
Democratizing access to cheap, readily available, and long DNA will facilitate an explosion in the bioeconomy; companies that synthesize DNA at a low cost, while still providing high accuracy and fast turnaround time, will create tremendous value. Historically, higher turnaround times, improved accuracy, and precipitous cost decreases in DNA synthesis have been made possible through engineering advances - we predict that this trend will continue in the future.
Context
DNA synthesis is a primary bottleneck in the near-future bioeconomy. Without rapid and high fidelity DNA writing technology, the promise of DNA storage, de novo organisms, and gene therapy cannot be realized. Where DNA sequencing has decreased by six orders of magnitude (in light of the $100 Ultima genome, although there is some skepticism). DNA synthesis has lagged behind, only decreasing in cost by 3-4 orders of magnitude. (The cost of DNA synthesis varies depending on the method with a trade-off in cost an accuracy - Table 1.) This is due to the comparative difficulty in having a template (sequencing) or not (synthesis). De novo DNA synthesis first requires the synthesis of a single strand of DNA which can be conducted through phosphoramidite chemistry or template-free DNA synthesis enzymes. Both techniques require the sequential addition of nucleotides.
The DNA synthesis market first took off when Twist was able to miniaturize the phosphoramidite chemistry on a silicon wafer (chronicled here). Twist’s current technology allows for the parallel synthesis of 9,500+ strands of DNA. Twist’s success hinges upon parallelization which drives down cost, improves quality, and increases length of constructs (and turnaround time if you’re a DNA service company). However, phosphoramidite chemistry is still limited in sequence length (200-mers), accuracy and yield, due to imperfect nucleotide coupling efficiency, imperfect protection/deprotection chemistry, and depurination occurring as a natural side reaction. That being said, there are depurination-resistant methods and reagents which could theoretically allow for longer sequence lengths with phosphoramidite chemistry.
Figure 1: Multiplexed DNA synthesis with Twist Biosciences.
Chemical Oligonucleotide Synthesis Background
Before we can think about synthesizing DNA or even full-length genes, we must first understand how to synthesize shorter sequences of nucleotides - called oligonucleotides, or oligos for short. Oligos can be used in a variety of applications, such as R&D, disease diagnosis, or even therapeutics. Like most discoveries in science, development of the phosphoramidite chemistry to pioneer synthesis of oligonucleotides was based on the contributions of numerous scientists over decades of work, with an exciting history. Although hard to pinpoint, one could argue the first direct contribution came from Nobel laureate Alexander Todd’s research group in the 1950s. Following Todd and coworkers’ contributions, research groups of Har Gobind Khorana, Robert Letsinger, Marvin Caruthers, and others continued to push the boundaries of oligonucleotide synthesis. Today, much of the chemistry used in industry is heavily based on the work discovered between the 1970s and 1980s.
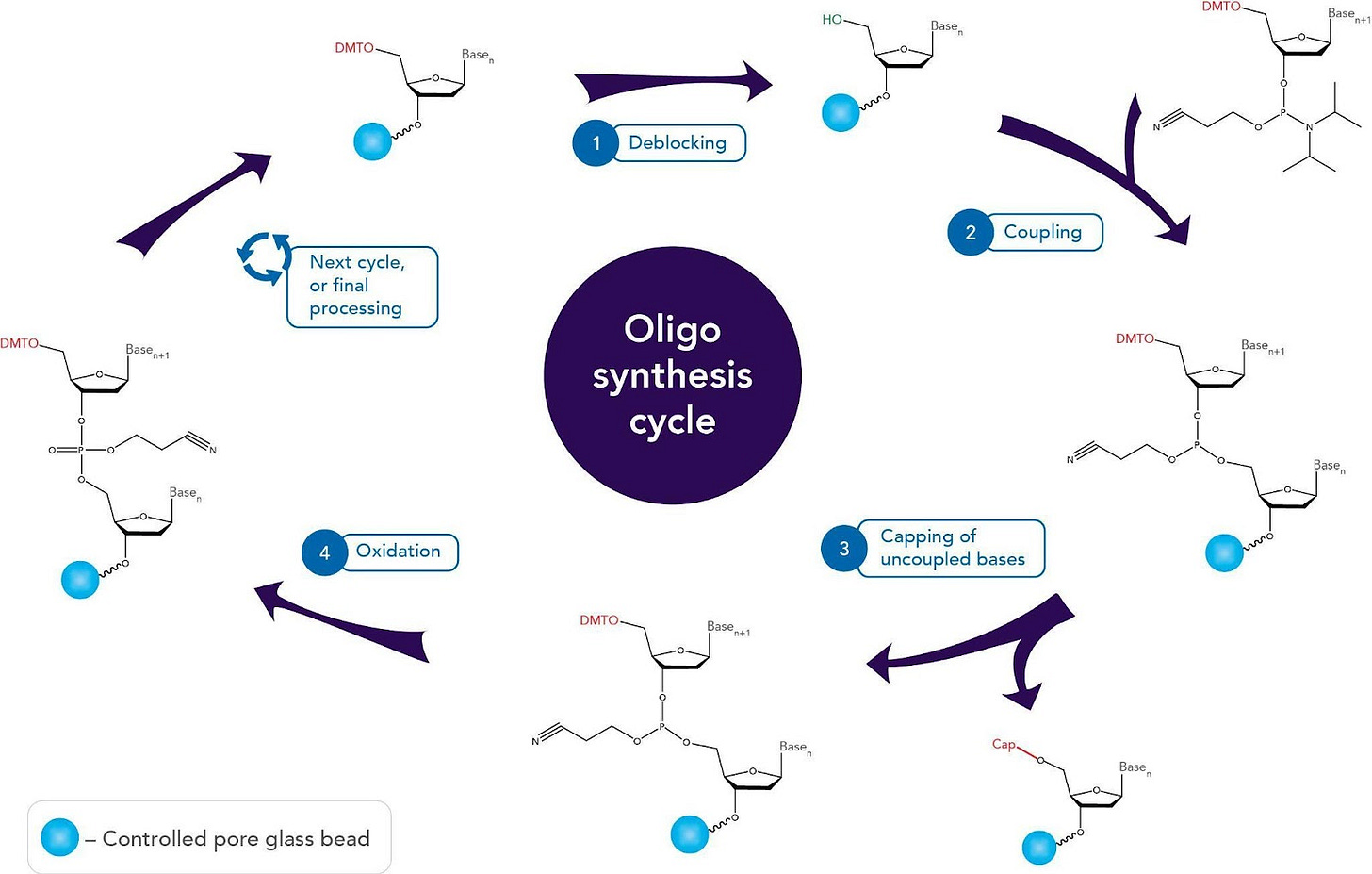
Oligonucleotide synthesis for both DNA and RNA is carried out by a stepwise addition of nucleotide residues through a cyclic process of four steps: 1) de-blocking (detritylation), 2) coupling, 3) capping, and finally 4) oxidation. Although reagent-based improvements have been made since initial discovery of phosphoramidite chemistry - such as increasing stability and allowing for storage of intermediates by swapping out a chloride leaving group for an amine-based leaving group - the largest improvements have come through engineering-focused approaches. The first major engineering improvement came through using a solid-phase support to carry out the synthesis. This process of solid-phase synthesis involves tethering the first nucleotide via its 3'-terminal hydroxyl group to an insoluble solid like a glass bead, and continuing to add sequentially to that first tethered nucleotide. This provides multiple advantages like allowing for impurities and excess reagents to easily be washed away, while keeping only the desired oligos that are tethered to the support. This also allows large ratios of reagents to be used to drive the oligo synthesis to completion more quickly through improving the kinetics. However, possibly most importantly, using a solid-phase support empowers integration with technology and allows for automation of oligonucleotide synthesis. The introduction of automation in oligonucleotide synthesis was a giant step in democratization of science. The automation of DNA synthesis and subsequent cost reduction has enabled scientists to order genes online and have them delivered within a week! Although rooted in chemistry and biology, the revolution came through applying engineering and technology; a recurring theme in the world of improving biology and chemistry.
Figure 2: Stepwise oligonucleotide synthesis.
Pitfalls and Roadblocks in Chemical Synthesis
Although there have been major improvements in the world oligo synthesis and phosphoramidite chemistry, there are several large roadblocks preventing affordable and sustainable longer oligios and DNA. Fundamentally, chemistry is imperfect and 100% coupling efficiency is not possible. Even with extreme care, some oligos will not couple with the newly introduced nucleotide - causing short, prematurely truncated oligos. Another potentially detrimental scenario centers around improper protecting/deprotecting of the 5′ hydroxyl group on the sugar portion of the nucleotide. If this hydroxyl is not protected, an oligo with a “deletion” in its sequence can be produced (i.e. N-1 sized oligo). Additionally, larger oligos can undergo a common side reaction found in phosphoramidite chemistry that also wreaks havoc on oligos: depurination. Depurination, the loss of purine bases (adenine and guanine) from a nucleotide, creates an abasic site. This abasic site then reacts with the final alkaline nucleobase deprotection reagents and then also leads to prematurely truncated oligos forming (do you see a trend here?). As a result, this group of shortened “failed oligos'' accumulates, statistically speaking, with every added nucleotide, whether a function of a “low” coupling efficiency, imperfect protecting/deprotecting, or depurination side reactions. Applying this knowledge, we see that as oligo length increases, so does the total percentage of “failed oligo” products. This principle fundamentally limits the length of oligos that can be made with a high yield and accuracy through chemistry. Generally, it is accepted that through chemical means, oligonucleotides with a length between 120 to 200 nucleotides can be synthesized accurately with acceptable yield. Although the sizes of genes vary wildly, some genes reach 2 million nucleotides in length! We’ve come a long way, but if the goal is to eventually synthesize full genes (and organisms) with ease – we still have a way to go. In addition, phosphoramidite chemistry fundamentally uses large amounts of flammable organic solvents, like acetonitrile, which incurs high cost, creates potentially hazardous situations, and is largely harmful to the planet. These challenges have led other labs to look beyond chemistry for solutions.
Enzymatic DNA synthesis
Given the pitfalls of traditional chemical synthesis, enzymatic methods of DNA synthesis have been proposed as an alternative to phosphoramidite chemistry. The most common form of enzymatic DNA synthesis is terminal deoxynucleotidyl transferase (TdT), used in nature to add bases to B and T-cell receptor sequences. Like phosphoramidite chemistry, TdT requires sequential and controlled addition of nucleotide bases but has not reached the n-mer length of phosphoramadite synthesis. To add to this, there can also be complications with secondary structure of oligonucleotides but this can be controlled by increasing temperature to prevent secondary structure annealing. Secondary structure is harder to control with enzymatic synthesis because higher temperatures can decrease polymerization rate, or even lead to protein denaturation, which is not a problem in chemical synthesis. However, enzymatic synthesis has some advantages in speed and use of non-hazardous ingredients. DNA Script, Nuclera, Kern Systems, Ansa and Molecular Assemblies all use TdT technology. Other companies, such as SynHelix/Quantoom use DNA primase enzymes, while others employ proprietary geometric enzymatic synthesis instead (Camena Biosciences). After years of being a stalwart of the phosphoramidite platform, Twist has announced plans to develop its synthesis capabilities with enzymes. Although there is no commercially produced DNA from Twist synthesized with enzymes, the strategy expansion highlights the broader vision of DNA synthesis by Twist and may signal that enzymes are the future of DNA synthesis. Namely, Twist dovetails its goals in DNA storage with enzymatic DNA synthesis, invoking that the hazardous chemical used during phosphoramidite synthesis doesn’t align in a future with many decentralized DNA storage facilities.
Conclusion
The arrival of the bio-based future is dependent on the arrival of cheap, high quality, readily available DNA. Amazing progress has been made in oligo and polynucleotide synthesis from advances in chemical synthesis to capitalizing on biology. These advances, like others in the biological world, stem from solving “engineering challenges” rather than “biological challenges.” DNA is continuously being synthesized by humans, other animals, plants, fungi, microorganisms, and viruses. The big question is, when will our engineering advances be good enough to capitalize on what Mother Nature already does so well?
Some relevant DNA synthesis companies (mostly private companies)
Related reading
Acknowledgments
This article took many iterations between us (Nima and Shelby) and our respective teams at Breakout Ventures and Compound. We’re especially grateful to Lindy Fishburne and Michael Dempsey who worked to make this article the best it can be.





Hi,
Does this carry a Creative Commons Copyright so that others can use it in their teaching